Clinical trial diversity must improve to ensure drugs work for all. By Ben Hirschler.
The pharmaceutical industry has a problem. Every year companies spend billions of dollars on clinical trials to assess the efficacy and safety of medicines—yet for large sections of the populations they serve, the results are simply not good enough.
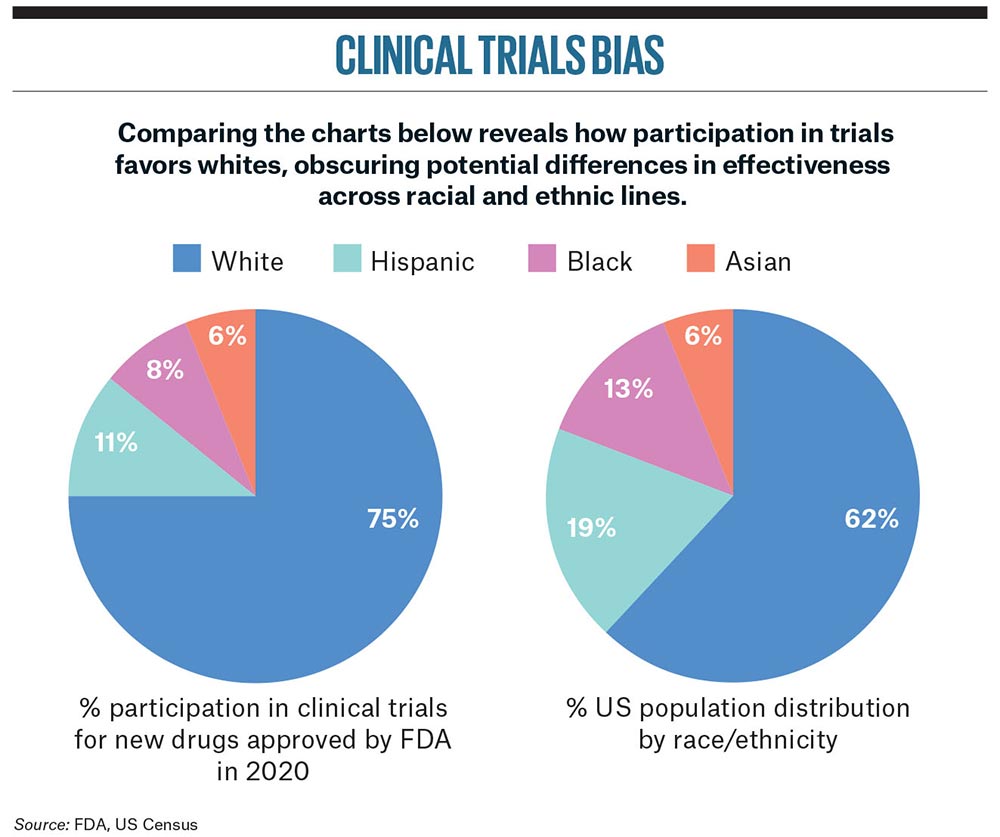
Clinical trials are falling short by routinely failing to accurately reflect the diversity of different patient groups. This means the data they generate is not painting the full picture of the good (and sometimes bad) that modern medicine can do.
Race, ethnicity, sex and age all affect how different people respond to the same medicine, vaccine or other medical intervention. Unless trial participants testing a new therapy are representative of those who will use it, there can be no certainty as to how it will work in medical practice.
What is more, physicians may be reluctant to prescribe new medicines to groups of people who were under-represented in clinical trials, resulting in the effective exclusion of those groups from the potential benefits of ground-breaking medical research.
Unfortunately, it is the most vulnerable individuals in society, especially those in non-white communities, who are the biggest losers since they are the ones who are most under-represented.
“The magnitude of the disparity is much greater than people realize, especially among patients who identify as Black,” said Professor Kenneth Getz, Executive Director of the Tufts Center for the Study of Drug Development in Boston, one of the world’s leading experts on clinical trial practices and trends.
“Any insights into the efficacy and the safety of a given treatment are sub-par until researchers have a deep understanding of the entire diverse population that might benefit from that therapy. Without it, our drug approval process is incomplete.”
US Food and Drug Administration figures show that 75% of participants in trials of recently approved drugs were white.
While the problem has been brewing for years, the disproportionate impact of the COVID-19 pandemic on minority communities has now thrown it into sharp focus. In addition, coming on top of the Black Lives Matter movement, the predominance of white participants in some early coronavirus vaccine trials has also attracted widespread criticism.
As a result, the clinical trials diversity gap is emerging as a critical issue for the pharmaceutical industry—and one with potentially far-reaching reputational implications for individual companies.
All those involved agree that ensuring the correct balance of different groups in trials is both a moral and a scientific imperative. Yet the evidence suggests there is still a long way to go.
US Food and Drug Administration figures show that 75% of participants in trials of recently approved drugs were white, even though minority racial and ethnic groups make up some 40% of the US population. The picture is similar for new medicines approved in Europe. A recent study by Getz and his team found that over half of drug trials supporting EU Commission approval under-represented non-white racial groups by more than 20%.
In some disease areas, the disparity is even starker. Black people accounted for only 2% of participants included in Alzheimer’s disease trials reported in the past decade, even though they are about twice as likely to have dementia as older white people, according to a Bloomberg News analysis.
Clearly, action is needed to redress the balance, given the fact that genetic and other differences can affect drug response to an extent that it impacts prescribing decisions. In the case of high blood pressure, UK national guidelines recommend avoiding certain drugs for people of African descent, although a lack of decisive evidence means opinion on this varies.
Several factors may account for medical differences between populations groups. Sometimes it is down to the way medicines are metabolized in the body, as with certain antidepressants and antipsychotics. In other cases, particular groups are more prone to a disease. Black individuals, for example, are more likely to have a gene called APOE4 that predisposes people to Alzheimer’s.
This makes it essential to drill down to specific therapeutic areas to truly understand the problem.
“The variation in disparity by disease condition is very significant,” said Getz. “We need to look at disparities by specific disease conditions to see the places where we could really try to target the solution.”
Multiple sclerosis and multiple myeloma—two diseases that disproportionately affect Black people—stand out as among the most problematic. A recent analysis by the IQVIA Institute for Human Data Science found that clinical trials in these two areas showed the greatest deviation from underlying US disease epidemiology, while migraine trials had the greatest alignment.
Getting the correct balance of trial participants is also a major challenge when it comes to gender. Although women now make up a higher percentage of clinical trial participants overall than men—accounting for 56% in the latest FDA data—they remain under-represented in certain key areas, such as heart disease.
So why is it so hard to get the diversity balance right?
It turns out there are multiple barriers to clinical trial participation, including lack of geographic access to study centers, limited awareness that research is taking place, time constraints due to employment or care commitments, cost considerations and language obstacles.
More fundamentally, in many minority communities there is also a lack of trust in the medical community and the drug development process due to past discrimination and mistreatment. This is exemplified, most notoriously, by the Tuskegee syphilis study that ran from 1932 to 1972, in which African American men were deliberately left untreated.
A 2021 survey of members of the Pharmaceutical Research and Manufacturers of America found that 61 percent of respondents had defined goals and objectives to enhance clinical trial diversity.
These days, pharmaceutical and biotechnology companies are paying a lot more attention to improving diversity in their clinical trials, while regulators on both sides of the Atlantic are also pushing the agenda forward.
A 2021 survey of members of the Pharmaceutical Research and Manufacturers of America found that 61% of respondents had defined goals and objectives to enhance clinical trial diversity—a promising sign of progress, but also an indication of the scale of the work that still needs to be done.
Ultimately, ensuring diversity in clinical trials is not only a matter of equity and good corporate practice, in line with environmental, social and governance (ESG) goals. It also makes sound business sense. “Doing good, robust science has market implications for companies because they will be reaching more people and delivering therapies that work better,” Getz said.
A range of solutions are now being explored to draw in more diverse pools of participants. Digital technology is part of the answer, with new tools such as wearable devices and telemedicine allowing people to take part in so-called decentralized studies from their own homes. At the same time, the ability to mine electronic health records should make it easier to identify patients who may be suitable for specific trials.
But deeper cultural changes are also needed to overcome skepticism among certain communities. One way of achieving that is to increase the diversity of healthcare professionals involved in research. Getz’s team at Tufts found that sites with higher racial and ethnic diversity among medical staff members saw that strongly mirrored in the range of patients they enrolled.
“This is a holistic challenge,” Getz said. “Companies can promise until they are blue in the face that they are going to improve diversity, but if they don’t get the patient advocacy groups, the community leaders, the healthcare providers, the investigator sites, and everybody else on board then they will continue to fall short.”
More from this issue

Acceleration
Most read from this issue

The Storyteller





